
Ground-breaking research
Harnessing stromal signalling pathways to induce and potentiate anti-cancer immune responses.
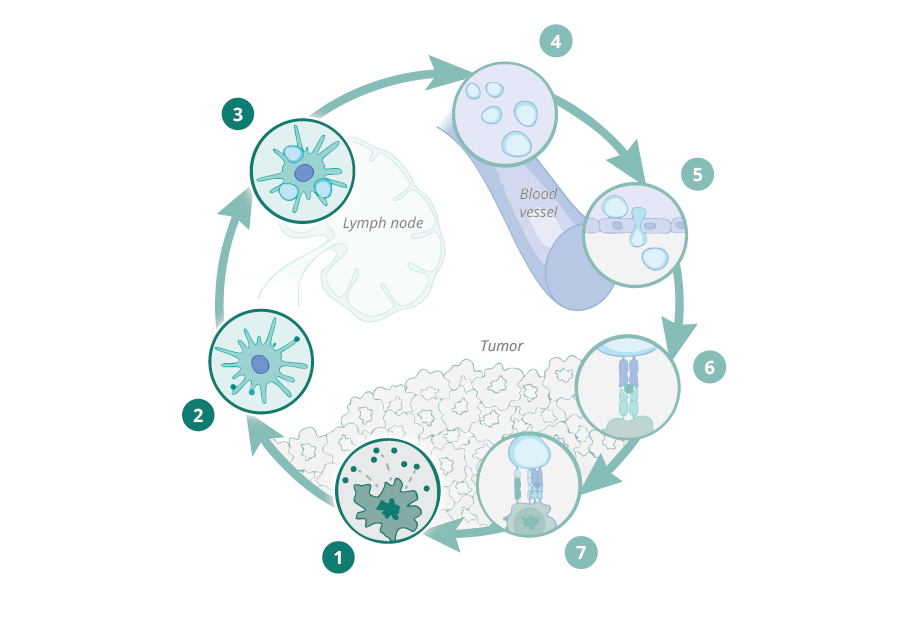
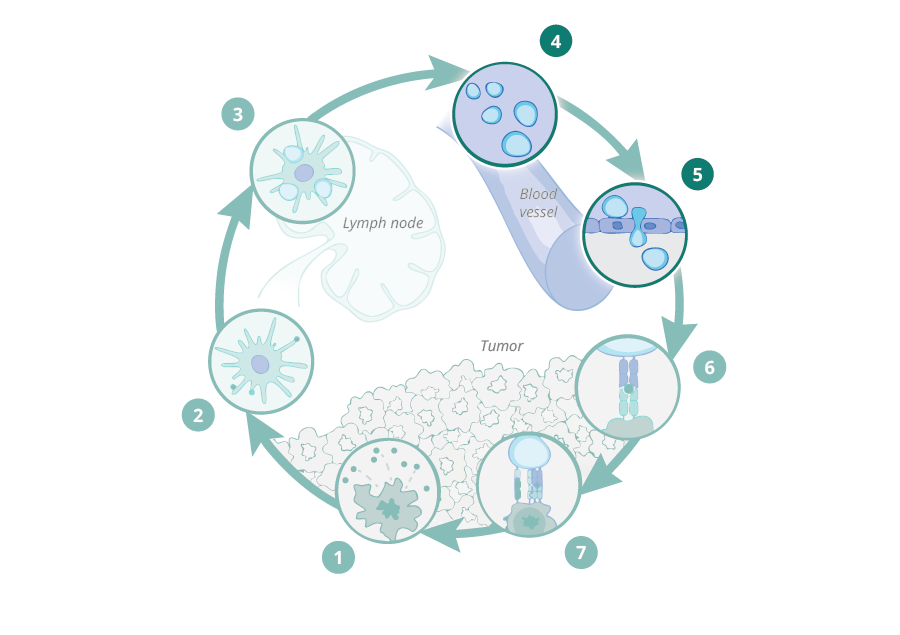
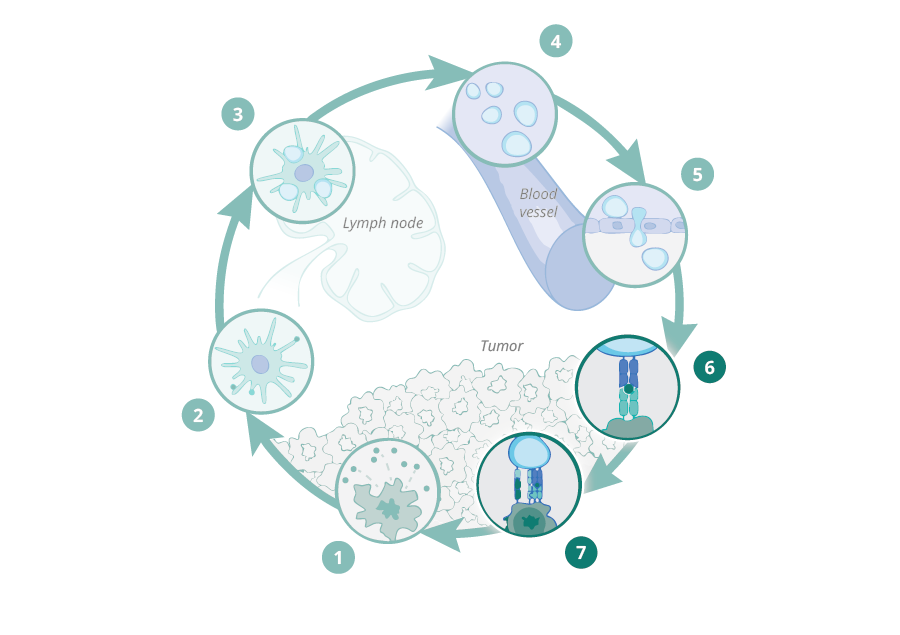
First and second DC:CD8+ T-cell "touches" are necessary for efficient tumor cell killing by tumor-specific T-cells



Sub Heading 2
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

Sub Heading
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

Sub Heading 3
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Our Technology



Our Story
During 10 years of research, we have demonstrated that stromal proteolysis of the matrix proteoglycan versican (VCAN) generates a bioactive fragment, versikine, that has potent immunostimulatory properties, promoting T-cell infiltration into the tumor. Versican proteolysis likely triggers a dominant homeostatic host response, sensing tissue remodeling and aiming to restrict local tumor expansion through engagement of adaptive immunitv. The pathway is widely conserved in human cancers. Indeed, we have shown that stromal versican proteolysis correlates with T-cell infiltration into tumor nests in a wide spectrum of human cancers;
- multiple myeloma (Blood, 2016)
- colorectal cancer (J Immunol 2017)
- lung carcinoma (Cell Reports 2022) and
- breast cancer (Cancers, 2025)
Versikine is a powerful modulator of antigen presenting cell (APC) activity in the tumor microenvironment (TME), regulating the cross-talk between antigen presenting cells (APCs) and effector CD8+ T cells and its presence is associated with both activation of APCs and expansion of the effector T cell population as well as penetration into the tumor tissue. In vivo data confirm the importance of versikine generation in potentiating the effect of immune checkpoint inhibitors (ICIs), as well as synergizing with ICIs to produce tumor regression in multiple tumor models. We are now in a position to further characterize versikine and use it as a basis to develop anti-cancer therapeutics with potential broad utility in solid and liquid tumors.
